
Ending cancer with theranostics
Ending cancer with theranostics
Nuclear medicine is currently at the forefront of practice changing research and rapid growth in medicine. This speciality has been evolving since pioneering work in the 1930’s by Dr Saul Hertz, an American physician, using radio-active iodine for treating thyroid disease. In 1944, Dr Hertz said “My new research project is in cancer of the thyroid which I believe holds the key to the larger problem of cancer in general”. This vision has been realised with advances in positron emission tomography (PET) and radionuclide therapy. This ability to use a radionuclide for both imaging and therapy is shaping the future of personalised medicine. This is otherwise known as theranostics.

Dr Hertz administering radio-iodine for hyperthyroidism circa.1945
Theranostics offers an excellent opportunity for physicians to prepare themselves for the future of precision medicine. Nuclear medicine is a dynamic specialty with a dual training pathway through either basic physician training or radiology training. Both clinical and diagnostic skill sets are essential in molecular imaging and theranostics. Theranostics is a specialty proven to be adaptive to innovation and change. Despite having a promising and interesting career prospect, opportunities for medical students and trainees to be exposed to nuclear medicine has to date been limited, and awareness of this rapidly expanding hybrid specialty warrants greater attention.
Theranostics has become an internationally trending topic over recent years, and much of its advancement is being driven at a national level right here in Australia. While the term may resonate with new medicine, theranostics has been an integral part of nuclear medicine for many decades. The theranostics approach labels a ligand with radioactive nuclide with diagnostic and therapeutic properties, targeting a specific receptor or physiologic pathway.
In treatment of cancer, radionuclide therapy offers the precision killing of cancer cells at a whole-body scale with relatively limited toxicity. As new molecular targets are being discovered, there are unlimited possibilities.
Prostate cancer theranostics
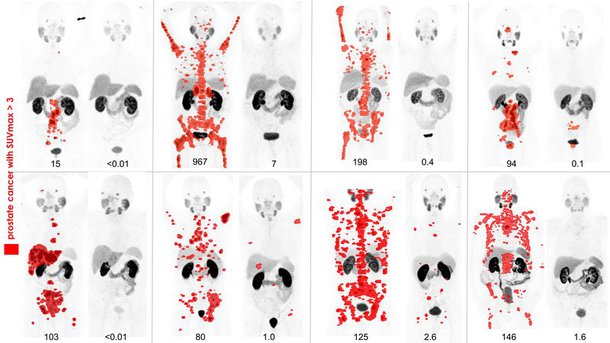
In the last six years, Australian researchers have led the application of radiolabelled prostate specific membrane antigen (PSMA), which is transforming the clinical landscape of prostate cancer management. This transmembrane glycoprotein is highly expressed in the majority of prostate cancer cells, providing a molecular target for diagnostic and therapeutic applications. A 10-site Australian multicentre, randomised study, the ProPSMA Trial, was published in The Lancet (April, 2020) demonstrating that Gallium-68 (68Ga)-PSMA PET/CT has superior diagnostic accuracy to conventional CT and whole body bone scan for staging men with newly diagnosed high-risk prostate cancer.
The network established for the ProPSMA trial led directly to another randomised trial in men with metastatic castration-resistant prostate cancer, called TheraP, comparing Lutetium-177 (177Lu)-PSMA theranostics to care cabazitaxel chemotherapy in 200 men with progressive metastatic castration resistant prostate cancer. The study results were presented at the world’s pre-eminent oncology conference ASCO20 and demonstrated a higher response rate and lower toxicity in the men receiving radionuclide therapy. These practice-changing results are paving the way for PSMA PET/CT and PSMA therapy to become a new standard-of-care option for men with prostate cancer.
Establishing a Centre of Excellence
To accelerate research and development, the Prostate Theranostics and Imaging Centre of Excellence (ProsTIC) has recently been established at the Peter MacCallum Cancer Centre. ProsTIC comprises a multidisciplinary team led by nuclear medicine physician Professor Michael Hofman and includes nuclear medicine, medical oncology, radiation oncology, urology and laboratory-based doctors and researchers with a strong patient-centered philosophy. Excitingly, nuclear medicine is at the core of this multidisciplinary team.
Dr Nattakorn Dhiantravan, an early career nuclear medicine physician, is the proud recipient of the RACP Arnott Research Entry Scholarship in Cancer Research. This is enabling him to undertake a PhD investigating the role of 177Lu-PSMA as a first-line rather than a last-line of therapy. He is running two clinical trials, one comparing the addition 177Lu-PSMA to docetaxel chemotherapy in men with newly diagnosed metastatic prostate cancer, and another examining the therapeutic activity of this novel agent in a neoadjuvant setting prior to radical prostatectomy. Dr Dhiantravan is also focusing on the discovery of novel molecular imaging biomarkers in prostate cancer, through integration of artificial intelligence applications.
“This scholarship, together with grant funding from Movember, is enabling me to pursue a combined clinical and research career. My daily work involves a balance between clinical, imaging diagnostic and research responsibilities as well as close collaboration with colleagues from medical oncology, urology, radiation oncology, radiochemistry, medical physics and imaging technology teams. Nuclear medicine has offered me an ideal environment and opportunity to grow as a physician and incorporate new and exciting discoveries into my daily practice,” said Dr Dhiantravan.
Contributors
Dr Nattakorn Dhiantravan, Professor Michael Hofman and
Ms Annette Van Der Heyden.
Funding
ProsTIC is funded by a grant from the Prostate Cancer Foundation (PCF) and the Peter MacCallum Foundation. UpFrontPSMA is funded by a grant from Movember and Medical Research Future Fund (MRFF). Find out more at http://petermac.org/prostic
L-R Dr Dhiantravan and Professor Hofman